
Fruit Battery Experiment
Using PASCO’s wireless voltage and current sensors, we conducted the fruit battery experiment. We tested 7 different kinds of produce to try and determine which one had the highest electric charge, and would make the best battery.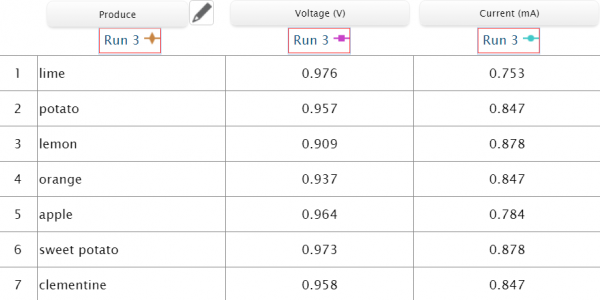
With these sensors you are able to chart and record voltage and current data right in SPARKvue! Then you can compare and contrast the data recorded across multiple different kinds of produce.
Using copper wire and a zinc coated nail, we connected an orange, clementine, lemon, lime, potato, sweet potato and apple to the sensors and charted their volts and milliamps.
We hypothesized that the lemon would have the highest voltage, due to the fact that it is an extremely acidic fruit. The lime is also an acidic fruit, so that was an alternate option that was considered. Due to the fact that the lemon was a larger fruit, and would maybe be able to hold more charge, we picked the lemon for our theorized winner.
 We set the voltage and current sensors to the manual data sampling option so that we could choose when to record data and input it into the table when the voltage and amperage stabilized. We then made an incision in the fruits to insert the copper wire, and pushed the zinc coated screw into the produce. With the citrus fruits, we needed to roll them on the table to get the juices flowing inside of it and gain more acid for the zinc to interact with, making the battery stronger. After connecting the sensors to SPARKvue, and connecting the negative and positive wires to their proper nodes, we were able to record data for all 7 items into the chart. With SPARKvue we could measure, record, and compare data all in one place.
We set the voltage and current sensors to the manual data sampling option so that we could choose when to record data and input it into the table when the voltage and amperage stabilized. We then made an incision in the fruits to insert the copper wire, and pushed the zinc coated screw into the produce. With the citrus fruits, we needed to roll them on the table to get the juices flowing inside of it and gain more acid for the zinc to interact with, making the battery stronger. After connecting the sensors to SPARKvue, and connecting the negative and positive wires to their proper nodes, we were able to record data for all 7 items into the chart. With SPARKvue we could measure, record, and compare data all in one place.
What may seem like similar results between all the produce is far from ambiguous as by nature the results will be similar between the produce, but there are still outliers that stand out. This experiment allows students to engage in a fun activity by hypothesizing which produce would be the best battery. It makes for a perfect opportunity for your student scientists to strengthen their critical thinking skills and increase their scientific knowledge on electricity!
To advance upon this already fascinating experiment, try connecting an LED in series or in parallel, and see how many fruits it takes to light it up! Have students experiment and determine the best approach to make the LED nice and bright! You can connect multiple LEDS, and multiple fruits, and even compare your voltage and amperage after powering an LED to see if anything has changed!
How The Battery Works
The specifics of the science behind fruit batteries is similar for all types of produce, in using the transfer of negative electrons for creating an electrical current. Specifically in the case of the lemon, it reacts with the zinc and loosens electrons. Copper pulls electrons more strongly than zinc, so negative electrons will move towards the copper when the electrodes are connected by wires, while the positive electrons remain inside the fruit. Moving electrons are called an electric current, so when the electrons move through the wires a charge is detected!
Materials Used:
- PS-3212 Wireless Current Sensor
- PS-3211 Wireless Voltage Sensor
- Fruit Battery Experiment Guide
- Lemon, Lime, Orange, Clementine, Apple, Potato, Sweet Potato
- Copper Wire
- Zinc Coated Nail
